Shear Rate & Sink Conditions
Shear Rate
This is a very complex relationship but includes the interface between the surface of the solid and the rate at which fresh solvent contacts it.
If a tablet particle were to be suspended in media with no agitation at all, the liquid immediately around the tablet would become saturated and dissolution would essentially stop.
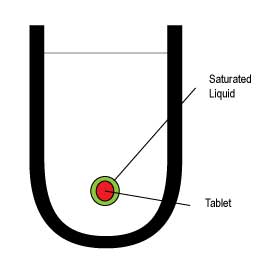
As the media starts to move then the saturated film is ‘washed’ away and new media enables the dissolution to continue again. Logically therefore, anything that affects the fluid dynamics or the way in which a dosage form disintegrates and dissolves should be understood and controlled
The Shear Rate depends on many variables including flow pattern variables, turbulence, viscosity, surface tension and dissolved gasses, which are in turn effected by other system variables to do with physical parameters.
Before any of those can be examined however, it is essential to ensure that there is sufficient media present to allow free dissolution of the active ingredient into solution.
Sink Conditions
If you put a spoon of sugar into a beaker of water it will dissolve readily. A second spoon will also dissolve. But keep adding spoonfuls and it becomes slower for the sugar to dissolve until at some point it becomes impossible for any more to dissolve as the solution becomes saturated.
Relating this to the dissolution of drugs, it is essential that as a drug dissolves, the presence of the already dissolved drug in solution should not affect the ability of more drug to be dissolved in any way. i.e. the concentration of drug in solution should not be anywhere other than the bottom of the saturation curve for that drug. Concentration should never be close to the saturation point.
If the concentration level were to rise too high, the dissolution rate of additional drug would be slowed and the data would cease to be reproducible.
In order to ensure that sufficient media is present in relation to the drug to be dissolved, typically 5 to 10 times greater volume of media is used in respect to that saturation point at which dissolution would slow. This is known as Sink Conditions – sufficient media to ensure un-impaired dissolution.
This is typically why dissolution is performed in larger volumes such as 900ml or 1litre. 500ml tests may be used where sink conditions permit and the measurable level of the drug is lower. In recent years, the introduction of microcapsules and very low dosage levels have led to mini vessel tests in volumes as low as 100mls or 200mls, but in all these cases, sink conditions are maintained. Conversely, if 1000mls is not enough volume, then larger 2000ml vessels can be used, and above that volume USP4 can be considered.
Next Steps
Having examined some of the basic theory behind dissolution, it is now possible to look at some of the practical issues and the effects that they can have on the dissolution profile. For the purposes of this tutorial we will concentrate on the two most common apparatus1 and apparatus 2, the rotating basket and paddle.


